No
magic bullet yet nor is any likely in the short term but lots of promising work
By
Will Collette
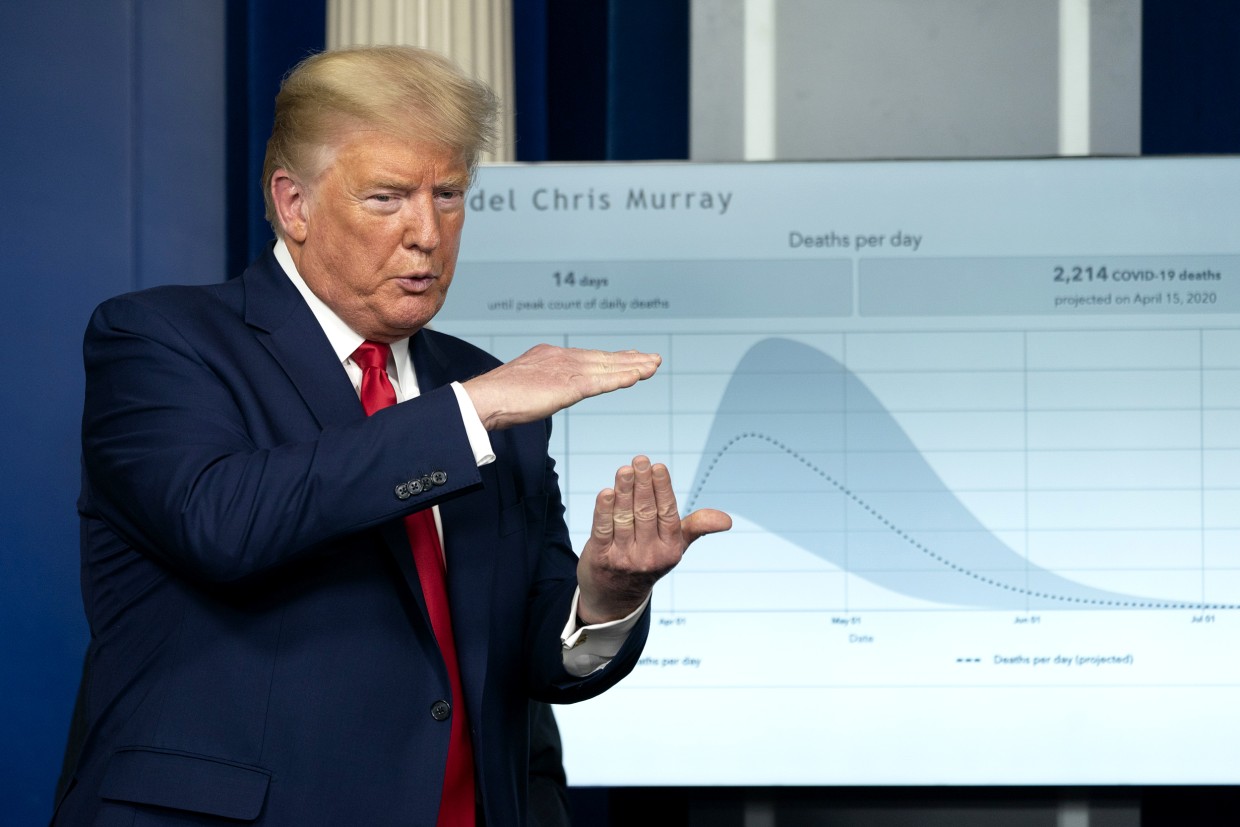 |
| Donald wants a magic cure. |
They
are making progress, though no serious scientist claims a quick and easy fix.
As they begin to publish their reports so their peers can begin to review and
test their findings, many of these reports find their way to the Science Daily.
I’ve
been visiting the Science Daily website for years because I like their mix of
report summaries from across just about every scientific discipline. With the
onset of COVID-19, it’s become essential daily reading.
I
also like Science Daily because it’s an open source. I have often re-published
material in Progressive Charlestown such as the three reports that follow.
They
are pretty good examples of the kind of COVID-19 research going on. There’s one from
England, another from the US and a third from Canada. They describe their
methodology and their findings in language that is about as clear as you can
expect from real scientists.
THIS
is the kind of work that will help us endure and prevail over this pandemic.
Here’s
the first of three.
Lancaster University
Summary:
A statistician who worked on the first published large
randomized clinical trial for a potential treatment for the COVID-19 virus said
the study produced positive results.
A Lancaster University statistician who worked on the first
published large randomised clinical trial for a potential treatment for the
COVID-19 virus said the scientific community was coming together to combat the
coronavirus.
There are currently no specific treatments for COVID-19.
However, it is possible that some existing drugs, usually used for other
conditions, may have some benefits.
Professor Thomas Jaki, from the Medical and Pharmaceutical
Statistics Research Unit in the Department of Mathematics and Statistics at
Lancaster University, said the initial trial investigated whether anti-viral
drugs used to treat HIV would relieve the symptoms of COVID-19 -- and started
when there were less than 500 confirmed cases worldwide.
The trial saw 199 COVID-19 patients at Jin Yin-Tan Hospital
treated either using standard of care or being given lopinavir-ritonavir. The
results were published recently and showed enough promise that further, larger
trials of the lopinavir-ritonavir treatment have now been established.
Specifically, the trial showed that patients who were randomly
chosen to receive lopinavir-ritonavir appeared to improve faster. Meanwhile,
among those patients who actually received lopinavir-ritonavir, the time to
clinical improvement was significantly shorter than in patients receiving
standard of care alone. At the same time acceptable safety levels were
observed.
Professor Jaki said: "The results were quite encouraging,
which has led to further studies taking place and I would expect to see these
treatments to be introduced into routine care, in some cases, in the coming
weeks."
The findings from this study will also be considered as part of
the forthcoming Randomised Evaluation of COVid-19 thERapY (RECOVERY) trial.
This will provide a trial platform to evaluate some of the approximately 30
treatments which are currently believed to have potential for treatment of
COVID-19.
The chief investigator for that trial is Peter Horby, Professor of Emerging Infectious Diseases and Global Health in the Nuffield Department of Medicine at the University of Oxford -- who was also part of the initial study in Wuhan.
The chief investigator for that trial is Peter Horby, Professor of Emerging Infectious Diseases and Global Health in the Nuffield Department of Medicine at the University of Oxford -- who was also part of the initial study in Wuhan.
Professor Jaki said the fact these studies were taking place
during a medical emergency had seen a determination to move things forward at
pace.
He added: "The astonishing thing about the RECOVERY trial
is the speed with which it got off the ground, from the initial agreement being
received from the Department of Health and Social Care it was just nine days
before the first patient was recruited. That process would usually take between
six and nine months.
"We have benefitted by learning from trials in China --
keep it simple. RECOVERY uses a very short protocol and attempts to minimise
burden on staff in hospitals which are overwhelmed, so we have tried to ensure
data collection is simple. It is an important study and one which needs to be
done quickly.
"This is one of the largest trials into treatments for
COVID-19. There is a second large trial that is currently recruiting in the UK
-- REMAP-CAP -- which is looking at impacts of treatments on severely ill
patients, but both teams have worked together to ensure they will be able to
learn as much as they can from both trials.
"This is unusual but a lot of the competitive nature of
these things isn't there at the moment. We all have the same goal."
The RECOVERY trial has been classed as an Urgent Public Health
Research Study. It is one of a round of projects to receive £10.5m as part of
the £20m rapid research response funded by UK Research and Innovation, and by
the Department of Health and Social Care through the National Institute for Health
Research (NIHR).
Chief Medical Officer Professor Chris Whitty and NHS England
Medical Director Professor Stephen Powis have written to NHS trusts in England
asking them to fully support the new trial.
Professor Jaki is also one of the authors of an opinion piece
published in the New England Journal of Medicine last week
on an approach to clinical trials around large epidemics.
While the piece was predominantly written months before the
coronavirus outbreak, it encourages greater collaboration between scientists
and much of the advice is already being reflected in the World Health
Organisation's developing strategy.
Professor Jaki added: "This is the culmination of a
conversation which started several years ago where the WHO recognised the
necessity of pre-planning in the event of a disease outbreak.
"While it may sometimes feel like we are unprepared for
incidents like the COVID-19 outbreak there is actually a co-ordinated global
effort among scientists, and Lancaster plays its part in that."
COVID-19
vaccine candidate shows promise, research shows
University of
Pittsburgh
Summary:
Scientists have announced
a potential vaccine against SARS-CoV-2, the new coronavirus causing the
COVID-19 pandemic. When tested in mice, the vaccine -- delivered through a
fingertip-sized patch -- produces antibodies specific to SARS-CoV-2 at
quantities thought to be sufficient for neutralizing the virus.
University of
Pittsburgh School of Medicine scientists today announced a potential vaccine
against SARS-CoV-2, the new coronavirus causing the COVID-19 pandemic. When
tested in mice, the vaccine, delivered through a fingertip-sized patch,
produces antibodies specific to SARS-CoV-2 at quantities thought to be sufficient
for neutralizing the virus.
The paper appeared
today in EBioMedicine, which is published by The Lancet,
and is the first study to be published after critique from fellow scientists at
outside institutions that describes a candidate vaccine for COVID-19. The
researchers were able to act quickly because they had already laid the
groundwork during earlier coronavirus epidemics.
"We had previous
experience on SARS-CoV in 2003 and MERS-CoV in 2014. These two viruses, which
are closely related to SARS-CoV-2, teach us that a particular protein, called a
spike protein, is important for inducing immunity against the virus. We knew
exactly where to fight this new virus," said co-senior author Andrea
Gambotto, M.D., associate professor of surgery at the Pitt School of Medicine.
"That's why it's important to fund vaccine research. You never know where the next pandemic will come from."
"That's why it's important to fund vaccine research. You never know where the next pandemic will come from."
"Our ability to
rapidly develop this vaccine was a result of scientists with expertise in
diverse areas of research working together with a common goal," said
co-senior author Louis Falo, M.D., Ph.D., professor and chair of dermatology at
Pitt's School of Medicine and UPMC.
Compared to the
experimental mRNA vaccine candidate that just entered clinical trials, the
vaccine described in this paper -- which the authors are calling PittCoVacc,
short for Pittsburgh Coronavirus Vaccine -- follows a more established
approach, using lab-made pieces of viral protein to build immunity. It's the
same way the current flu shots work.
The researchers also
used a novel approach to deliver the drug, called a microneedle array, to
increase potency. This array is a fingertip-sized patch of 400 tiny needles
that delivers the spike protein pieces into the skin, where the immune reaction
is strongest. The patch goes on like a Band-Aid and then the needles -- which
are made entirely of sugar and the protein pieces -- simply dissolve into the
skin.
"We developed this to build on the original scratch method used to deliver the smallpox vaccine to the skin, but as a high-tech version that is more efficient and reproducible patient to patient," Falo said. "And it's actually pretty painless -- it feels kind of like Velcro."
The system also is
highly scalable. The protein pieces are manufactured by a "cell factory"
-- layers upon layers of cultured cells engineered to express the SARS-CoV-2
spike protein -- that can be stacked further to multiply yield. Purifying the
protein also can be done at industrial scale.
Mass-producing the microneedle array involves spinning down the protein-sugar mixture into a mold using a centrifuge. Once manufactured, the vaccine can sit at room temperature until it's needed, eliminating the need for refrigeration during transport or storage.
Mass-producing the microneedle array involves spinning down the protein-sugar mixture into a mold using a centrifuge. Once manufactured, the vaccine can sit at room temperature until it's needed, eliminating the need for refrigeration during transport or storage.
"For most
vaccines, you don't need to address scalability to begin with," Gambotto
said. "But when you try to develop a vaccine quickly against a pandemic
that's the first requirement."
When tested in mice,
PittCoVacc generated a surge of antibodies against SARS-CoV-2 within two weeks
of the microneedle prick.
Those animals haven't
been tracked long term yet, but the researchers point out that mice who got
their MERS-CoV vaccine produced a sufficient level of antibodies to neutralize
the virus for at least a year, and so far the antibody levels of the SARS-CoV-2
vaccinated animals seem to be following the same trend.
Importantly, the
SARS-CoV-2 microneedle vaccine maintains its potency even after being
thoroughly sterilized with gamma radiation -- a key step toward making a
product that's suitable for use in humans.
The authors are now in
the process of applying for an investigational new drug approval from the U.S.
Food and Drug Administration in anticipation of starting a phase I human
clinical trial in the next few months.
"Testing in
patients would typically require at least a year and probably longer,"
Falo said. "This particular situation is different from anything we've
ever seen, so we don't know how long the clinical development process will
take. Recently announced revisions to the normal processes suggest we may be
able to advance this faster."
Additional authors on
the study are Eun Kim, Geza Erdos, Ph.D., Shaohua Huang, Thomas Kenniston,
Stephen Balmert, Ph.D., Cara Donahue Carey, Michael Epperly, Ph.D., William
Klimstra, Ph.D., and Emrullah Korkmaz, Ph.D., all of Pitt; and Bart Haagmans,
of Erasmus Medical Center.
Funding for this study was provided by National Institute of Allergy and Infectious Diseases grant R21-AI114264, National Institute of Arthritis and Musculoskeletal and Skin Diseases grants R01-AR074285, R01-AR071277 and R01-AR068249, and National Cancer Institute grant T32-CA175294.
Trial
drug can significantly block early stages of COVID-19 in engineered human
tissues
University of British
Columbia
Summary:
An international team
has found a trial drug that effectively blocks the cellular door SARS-CoV-2
uses to infect its hosts.
An international team
led by University of British Columbia researcher Dr. Josef Penninger has found
a trial drug that effectively blocks the cellular door SARS-CoV-2 uses to
infect its hosts.
The findings,
published today in Cell, hold promise as a treatment capable of
stopping early infection of the novel coronavirus that, as of April 2, has
affected more than 981,000 people and claimed the lives of 50,000 people
worldwide.
The study provides new
insights into key aspects of SARS-CoV-2, the virus that causes COVID-19, and
its interactions on a cellular level, as well as how the virus can infect blood
vessels and kidneys.
"We are hopeful
our results have implications for the development of a novel drug for the
treatment of this unprecedented pandemic," says Penninger, professor in
UBC's faculty of medicine, director of the Life Sciences Institute and the
Canada 150 Research Chair in Functional Genetics at UBC.
"This work stems
from an amazing collaboration among academic researchers and companies,
including Dr. Ryan Conder's gastrointestinal group at STEMCELL Technologies in
Vancouver, Nuria Montserrat in Spain, Drs. Haibo Zhang and Art Slutsky from
Toronto and especially Ali Mirazimi's infectious biology team in Sweden, who
have been working tirelessly day and night for weeks to better understand the
pathology of this disease and to provide breakthrough therapeutic
options."
ACE2 -- a protein on
the surface of the cell membrane -- is now at centre-stage in this outbreak as
the key receptor for the spike glycoprotein of SARS-CoV-2.
In earlier work, Penninger and colleagues at the University of Toronto and the Institute of Molecular Biology in Vienna first identified ACE2, and found that in living organisms, ACE2 is the key receptor for SARS, the viral respiratory illness recognized as a global threat in 2003.
His laboratory also went on to link the protein to both cardiovascular disease and lung failure.
In earlier work, Penninger and colleagues at the University of Toronto and the Institute of Molecular Biology in Vienna first identified ACE2, and found that in living organisms, ACE2 is the key receptor for SARS, the viral respiratory illness recognized as a global threat in 2003.
His laboratory also went on to link the protein to both cardiovascular disease and lung failure.
While the COVID-19
outbreak continues to spread around the globe, the absence of a clinically
proven antiviral therapy or a treatment specifically targeting the critical
SARS-CoV-2 receptor ACE2 on a molecular level has meant an empty arsenal for
health care providers struggling to treat severe cases of COVID-19.
"Our new study
provides very much needed direct evidence that a drug -- called APN01 (human
recombinant soluble angiotensin-converting enzyme 2 -- hrsACE2) -- soon to be
tested in clinical trials by the European biotech company Apeiron Biologics, is
useful as an antiviral therapy for COVID-19," says Dr. Art Slutsky, a
scientist at the Keenan Research Centre for Biomedical Science of St. Michael's
Hospital and professor at the University of Toronto who is a collaborator on
the study.
In cell cultures
analyzed in the current study, hrsACE2 inhibited the coronavirus load by a
factor of 1,000-5,000. In engineered replicas of human blood vessel and kidneys
-- organoids grown from human stem cells -- the researchers demonstrated that
the virus can directly infect and duplicate itself in these tissues.
This provides important information on the development of the disease and the fact that severe cases of COVID-19 present with multi-organ failure and evidence of cardiovascular damage. Clinical grade hrsACE2 also reduced the SARS-CoV-2 infection in these engineered human tissues.
This provides important information on the development of the disease and the fact that severe cases of COVID-19 present with multi-organ failure and evidence of cardiovascular damage. Clinical grade hrsACE2 also reduced the SARS-CoV-2 infection in these engineered human tissues.
"Using organoids
allows us to test in a very agile way treatments that are already being used
for other diseases, or that are close to being validated. In these moments in
which time is short, human organoids save the time that we would spend to test
a new drug in the human setting," says Núria Montserrat, ICREA professor
at the Institute for Bioengineering of Catalonia in Spain.
"The virus causing COVID-19 is a close sibling to the first SARS virus," adds Penninger. "Our previous work has helped to rapidly identify ACE2 as the entry gate for SARS-CoV-2, which explains a lot about the disease. Now we know that a soluble form of ACE2 that catches the virus away, could be indeed a very rational therapy that specifically targets the gate the virus must take to infect us. There is hope for this horrible pandemic."
This research was
supported in part by the Canadian federal government through emergency funding
focused on accelerating the development, testing, and implementation of
measures to deal with the COVID-19 outbreak.