By Stefanie Dodt and Jan Lukas Strozyk, ARD German TV, and Dara Lind in ProPublica,
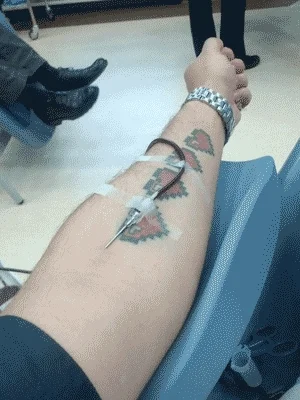 Every week, thousands of Mexicans cross the border into the U.S. on temporary visas to sell their blood plasma to profit-making pharmaceutical companies that lure them with Facebook ads and colorful flyers promising hefty cash rewards.
Every week, thousands of Mexicans cross the border into the U.S. on temporary visas to sell their blood plasma to profit-making pharmaceutical companies that lure them with Facebook ads and colorful flyers promising hefty cash rewards.The donors, including some who say the payments are their only income, may take home up to $400 a month if they donate twice a week and earn various incentives, including “buddy bonuses” for recruiting friends or family.
Unlike other nations that limit or forbid paid plasma donations at a high frequency out of concern for donor health and quality control, the U.S. allows companies to pay donors and has comparatively loose standards for monitoring their health.
Donating plasma too frequently can hurt a donor’s immune system. A donor’s level of the antibody immunoglobulin G should be screened every four months under guidelines from the U.S. Food and Drug Administration. But in the U.S., donors are still allowed to give plasma up to 104 times a year, far more than in most other countries. Selling plasma has been banned in Mexico since 1987.
Genesis, a 21-year-old Mexican studying to be a paramedic, who asked that her last name not be used for her protection, said she gives plasma twice a week in El Paso, Texas. She said she often faints, gets migraines and has numbness in her limbs. The more she donates plasma, the weaker she feels. “I have trouble lifting stuff, problems with my muscles.”
Like many Mexicans donors, Genesis comes into the U.S. on temporary visas, which allow non-immigrants to visit family, shop or “engage in commercial transactions, which do not involve gainful employment in the United States.”
The plasma companies contend their payments are not wages as defined under the law. They classify them as “compensation” for donor time, since the process often requires long waits and an hour or more hooked up to plasma extraction equipment.
“Plasma donors are compensated because of the time and commitment involved in being a regular plasma donor,” said the pharmaceutical company Grifols, which is based in Barcelona, Spain, and runs 17 plasma donation centers along the U.S.-Mexico border, more than any other company.
The companies also say they carefully monitor donors and follow all safety procedures. These flyers show that U.S.-based plasma centers accept Border Crossing Cards issued with temporary visas. The practice falls into a gray area of federal immigration law.
As the Trump administration clamps down on most traffic at the southern border, U.S. immigration agencies have done little to stop the stream of Mexicans using their B-1/B-2 visas to visit plasma centers.
In interviews with ARD German TV, some former plasma center employees said they routinely recommended that clients lie to U.S. Customs and Border patrol about the purpose of their U.S. visits.
“If people are using a B-1/B-2 visa to cross the border to sell plasma, they could be putting that document at risk,” said Roger Maier, a spokesman for CBP, the agency that examines visas at the border. “We strongly encourage people to not use their documents for that capacity.”
Maier said agents “have a lot of discretion in our ability to allow people to enter the United States based on the documents they present.” Asked if the use of visitor visas to donate plasma violates the law, he said, “I’m sorry, it’s a gray area, but I can’t give you a yes or no.”
The U.S. State Department said later that the visa rules do not address “the legality of this specific purpose of travel.” The Department of Justice and the U.S. Attorney’s Office for the Western District of Texas, which includes El Paso, did not respond to requests for comment.
The U.S. is the largest supplier of blood plasma in a $21 billion global market. FDA data shows that of the 805 plasma donation centers in the U.S., 43 are located along the southern border, up to 62 miles from Mexico.
The border clinics are the most productive, according to internal Grifols documents obtained by ARD. While most U.S. centers receive around 1,000 paid donations a week, centers at the border count more than 2,300. The documents show that border centers also rank highest in donor frequency; they top of list of centers with customers who donate 75 times or more per year.
Companies say they follow numerous safety precautions and seek new donors because they face a critical need as demand increases for lifesaving pharmaceutical products made from plasma, a yellowish fluid extracted from blood that contains antibodies that defend against disease.
The centers are mainly owned by Grifols, the Australia-based CSL and BPL, an emerging player headquartered in the U.K. U.S.-based GCAM Inc. has four centers along the southern border.
The FDA requires companies to monitor patient health before each donation. In some CSL centers, the payment amount depends on body weight, which determines how much blood plasma can be collected.
Donors weighing between 110 and 149 pounds receive $20 per donation, while donors between 175 and 400 pounds earn up to $40. However, a person who doesn’t finish the donation for any reason doesn’t get paid.
Calculating the exact number of Mexican donors coming into the U.S. is difficult because the companies do not disclose this data to state or federal agencies. ARD German TV obtained two weeks of donation counts for all the Grifols facilities in the U.S. from earlier this year, including those along the Texas-Mexico border.
Grifols employees at five centers along the border, who requested anonymity to protect their jobs, estimated that Mexican citizens make up 60% to 90% of donors each day, depending on the facility.
Based on those estimates, nearly 10,000 Mexican citizens donated plasma at those five Grifols centers during each of the two weeks. Thirty-eight additional plasma donation centers operate in the border area.
Reporters from ARD German TV and Searchlight New Mexico, an investigative news organization, talked to more than 50 plasma donors from the Mexican cities of Ciudad Juárez, Nuevo Laredo, Reynosa, Nogales and Matamoros, and they found that most use their plasma payments to cover basic needs like food, electricity, diapers and clothes. A typical worker in Ciudad Juárez makes about $9 a day.
Genesis lives just across the border from El Paso. Selling blood plasma in the United States is her only income; she’s crossed the border twice a week for three years. Her father, Gamaliel, introduced her to making a living this way.
Genesis, like most Mexican plasma donors, relies on a B-1/B-2 temporary visa to gain entry. In July alone, the State Department issued or renewed 84,804 Border Crossing Cards for Mexican citizens.
Most donors interviewed by ARD and Searchlight said that they give CBP officials a reason for their trips that obscures the real purpose. Donors are anxious and uncertain, but willing to take the risk. “I understand it is not illegal,” Gamaliel said. “But, if you get to an officer in a bad mood, they could take your visa. So it’s better not to tell.”
The 44-year-old fitness trainer runs his own gym in Ciudad Juárez, but he hardly makes $100 a month. For nine years, he’s donated twice a week to cover his and his youngest daughter’s basic needs.
Genesis said she tells CBP she’s visiting an aunt who lives in the U.S., but she rarely does so; instead, she goes directly to the lab. For the petite student, the moment right before crossing the border into El Paso is the most stressful. “I can never be sure. Who will be the officer on duty, what will they say or think. There’s always a chance they don’t believe you.”
The subterfuge is necessary because, at best, crossing into the U.S. to donate plasma for money falls into a questionable area of the law. While neither the State Department nor CBP calls plasma donation payments illegal, the law doesn’t define specifically if visa rules prohibit them. There haven’t yet been any investigations or prosecutions of plasma labs.
Potential liability for the pharmaceutical companies is even muddier. U.S. Citizenship and Immigration Services, which regulates legal immigration into the U.S., says that companies could be subject to charges if they engage in a “pattern or practice of knowingly hiring unauthorized aliens,” including people who are in the U.S. legally but not legally authorized to work.
The Plasma Protein Therapeutics Association, a trade organization representing for-profit plasma-producing pharmaceutical companies in North America and Europe, said in an email that donors are not employees of plasma centers and “only a person lawfully permitted to be in the United States can be accepted as a donor.”
CSL and Grifols, the dominant companies at the U.S. border, declined interview requests but gave written statements. Grifols emphasized that “all donors, regardless of where they come from, must comply with all necessary health, regulatory and legal requirements to donate. There are no exceptions.”
CSL said the company “complies with all laws in the countries in which we operate.”
BPL said in a statement it was “following all applicable guidelines that exist to promote the safety of both plasma donors and the patients who use plasma derived therapeutics.”
GCAM did not reply to a request for comment.
Grifols offers bonuses to lure donors. Only if donors give at the maximum frequency allowed by FDA they receive the full payment.
Blood and vaccines rank among the most valuable U.S. exports. In 2018, the U.S. collected 41 million liters of plasma intended for the production of medicine, and almost half of that was shipped abroad.
Around 78% of blood plasma exported from the U.S. ended up in Germany, Spain and Austria, where Grifols, CSL and others operate large high-tech plasma processing plants. A large portion of the drugs produced are then reimported and sold in the U.S.
There is little information about the long-term consequences of frequent plasma donations. Some scientists argue that a donor’s antibodies should be tested after every fifth donation, and some European countries like Germany require this. But the FDA in a statement defended its requirement that levels be checked every four months, saying, “We recognize that regulators in other countries may reach different regulatory conclusions even when considering the same data.”
Genesis keeps losing weight, leaving her perilously close to the 110-pound minimum required for donation. To avoid getting turned away at the clinics for being underweight, which has happened in the past, Genesis said she regularly fools the scales by putting water bottles in her pockets. Her trick has never been noticed.
When our reporters asked Genesis to get her blood levels examined, the lab results confirmed what Genesis felt. The test showed a dangerously low immunoglobulin G level.
According to Paul Strengers of the International Plasma Fractionation Association, a trade group for not-for-profit collectors of blood plasma, the loss of antibodies can damage the immune system and lead to serious infections like pneumonia.
The doctor who conducted the blood tests suggested that she stop donating plasma until her body is fully recovered. But, Genesis said, “stopping is a luxury I cannot afford.”
ProPublica is a Pulitzer Prize-winning investigative newsroom. Sign up for The Big Story newsletter to receive stories like this one in your inbox.