Look to the immune system
Prakash Nagarkatti, University of South Carolina and Mitzi Nagarkatti, University of South Carolina
 |
| What would Darwin consider the best adaptation to protect against the coronavirus? rolbos |
Organisms with genes better suited to the environment are selected for survival and pass them to the next generation.
Thus, when a new infection that the world has never seen before erupts, the process of natural selection starts all over again.
In the context of the coronavirus pandemic, who is the “fittest”?
This is a challenging question. But as immunology researchers at the University of South Carolina, we can say one thing is clear: With no effective treatment options, survival against the coronavirus infection depends completely on the patient’s immune response.
We have been working on how the immune response is a double-edged sword – on one hand helping the host to fight infections, while on the other hand causing significant damage in the form of autoimmune diseases.
The two phases of the immune response
The immune response is like a car. To reach a destination safely, you need both an accelerator (phase 1) and a brake (phase 2) that are functioning well.
Failure in either can have significant consequences.
An effective immune response against an infectious agent rests in the delicate balance of two phases of action.
When an infectious agent attacks, the body begins phase 1, which promotes inflammation – a state in which a variety of immune cells gather at the site of infection to destroy the pathogen.
This is followed by phase 2, during which immune cells called regulatory T cells suppress inflammation so that the infected tissues can completely heal. A deficiency in the first phase can allow uncontrolled growth of the infectious agent, such as a virus or bacteria.
A defect in the second phase can trigger massive inflammation, tissue damage and death.
The coronavirus infects cells by attaching to a receptor called the angiotensin-converting enzyme 2 (ACE2), which is present in many tissues throughout the body, including the respiratory tract and cardiovascular system.
This infection triggers a phase 1 immune response, in which the antibody-producing B-cells pump out neutralizing antibodies that can bind to the virus and prevent it from attaching to ACE2. This inhibits the virus from infecting more cells.
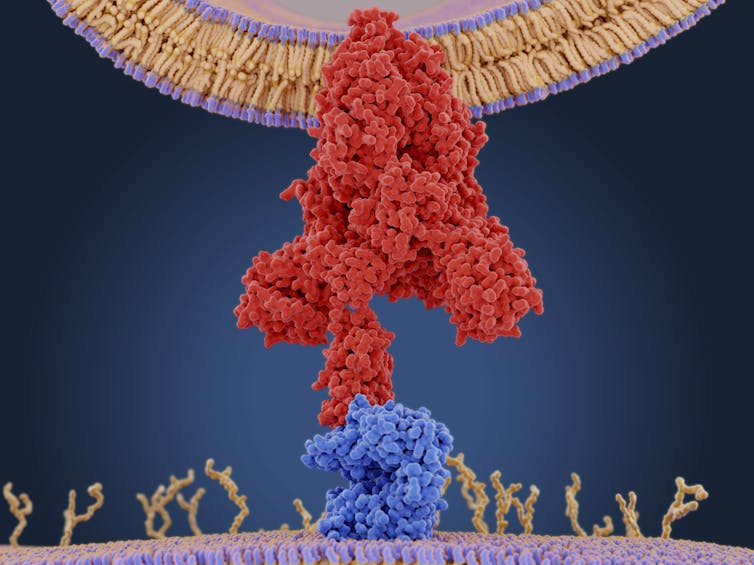 |
| Molecular model of a coronavirus spike (S) protein (red) bound to an angiotensin-converting enzyme 2 (ACE2) receptor (blue) on a human cell. Once inside the cell, the virus uses the cells’ machinery to make more copies of itself. JUAN GAERTNER/SCIENCE PHOTO LIBRARY |
If the immune system is compromised and works poorly during phase 1, the virus can replicate rapidly.
People with compromised immune systems include the elderly, organ transplant recipients, patients with autoimmune diseases, cancer patients undergoing chemotherapy and individuals who are born with immunodeficiency diseases.
Many of these individuals may not produce enough antibodies or killer T cells to counter the virus, which allows the virus to multiply unchecked and cause a severe infection.
Lung injury resulting from inflammation
Increased replication of SARS-CoV-2 triggers additional complications in the lungs and other organs.
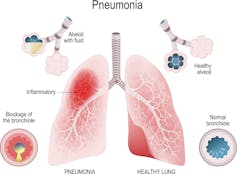 |
| When the alveoli, the location where oxygen is absorbed and carbon dioxide is expelled, is filled with liquid there is less space to absorb oxygen. ttsz / Getty Images |
However, as the coronavirus spreads, it is likely that the infection and the inflammation that ensues will disrupt this balance, allowing harmful bacteria present in the lungs to dominate.
This leads to development of pneumonia, in which the lungs’ air sacs, called alveoli, get filled with fluid or pus, making it difficult to breathe.
This triggers additional inflammation in the lungs, leading to Acute Respiratory Distress Syndrome (ARDS), which is seen in a third of COVID-19 patients.
The immune system, unable to control viral infection and other emerging pathogens in the lungs, mounts an even stronger inflammatory response by releasing more cytokines, a condition known as “cytokine storm.”
At this stage, it is also likely that the phase 2 immune response aimed at suppressing inflammation fails and can’t control the cytokine storm.
Such cytokine storms can trigger friendly fire – destructive, corrosive chemicals meant to destroy infected cells that are released by the body’s immune cells which can lead to severe damage to the lungs and other organs.
Also, because ACE2 is present throughout the body, the killer T cells from phase 1 can destroy virus-infected cells across multiple organs, causing more widespread destruction. Thus, patients that produce excessive cytokines and T cells can die from injury not only to the lungs but also to other organs such as the heart and kidneys.
The immune system’s balancing act
The above scenario raises several questions regarding prevention and treatment of COVID-19.
Because the majority of people recover from coronavirus infection, it is likely that a vaccine that triggers neutralizing antibodies and T cells to block the virus from getting into the cells and replicate is likely to be successful. The key to an effective vaccine is that it doesn’t trigger excessive inflammation.
Additionally, in patients who transition to a more severe form such as ARDS and cytokine storm, which is often lethal, there is an urgent need for novel anti-inflammatory drugs.
These drugs can broadly suppress the cytokine storm without causing excessive suppression of immune response, thereby enabling the patients to clear the coronavirus without damage to the lung and other tissues.
There may be only a narrow window of opportunity during which these immunosuppressive agents can be effectively used.
Such agents should not be started at an early stage of infection when the patient needs the immune system to fight the infection, but it cannot be delayed too long after ARDS development, when the massive inflammation is uncontrollable.
This window of anti-inflammatory treatment can be determined by monitoring the antibody and cytokine levels in patients.
With COVID-19, then, the “fittest” are individuals who mount a normal phase 1 and phase 2 immune response. This means a strong immune response in phase 1 to clear the primary coronavirus infection and inhibit its spread in the lungs.
Then this should be followed by an optimum phase 2 response to prevent excessive inflammation in the form of “cytokine storm.”
Vaccines and anti-inflammatory treatments need to carefully manage this delicate balancing act to be successful.
With this coronavirus, it isn’t easy to know who are the fittest individuals. It isn’t necessarily the youngest, strongest or most athletic individuals who are guaranteed to survive this coronavirus.
The fittest are those with the “right” immune response who can clear the infection rapidly without mounting excessive inflammation, which can be deadly.
[Get facts about coronavirus and the latest research. Sign up for The Conversation’s newsletter.]
Prakash Nagarkatti, Vice President for Research and Carolina Distinguished Professor, University of South Carolina and Mitzi Nagarkatti, SmartState Endowed Chair of Center for Cancer Drug Discovery, Carolina Distinguished Professor and Chair, Dept. of Pathology, Microbiology and Immunology, University of South Carolina
This article is republished from The Conversation under a Creative Commons license. Read the original article.