Anti-diarrhoea drug drives cancer cells to cell death
Goethe University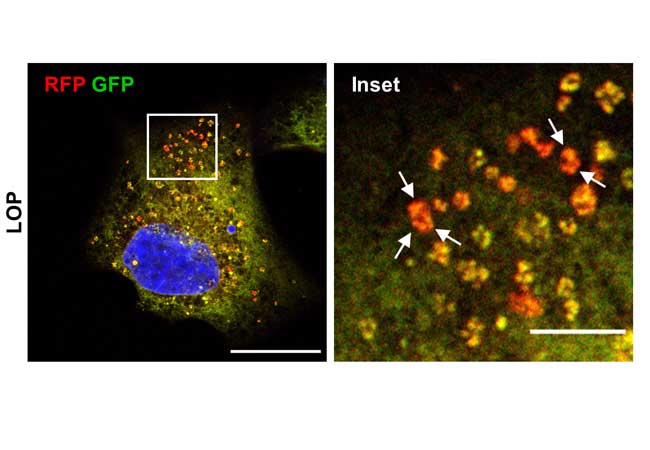 In
glioblastoma cells, the antidiarrheal drug loperamide triggers the degradation
of the endoplasmic reticulum. In the normal state, it is coloured yellow in
these microscopic images. In the degradation state, it glows as a red signal
(marked with arrows). Left scale bar: 20 µm, right scale bar (inset): 5 µm
(Photos: Svenja Zielke et. al.)
In
glioblastoma cells, the antidiarrheal drug loperamide triggers the degradation
of the endoplasmic reticulum. In the normal state, it is coloured yellow in
these microscopic images. In the degradation state, it glows as a red signal
(marked with arrows). Left scale bar: 20 µm, right scale bar (inset): 5 µm
(Photos: Svenja Zielke et. al.)
In cell culture, loperamide, a drug commonly used against diarrhoea, proves effective against glioblastoma cells.
A research team at Goethe University has now
unravelled the drug’s mechanisms of action of cell death induction and –
in doing so – has shown how this compound could help attack brain tumours that
otherwise are difficult to treat.
The research group led by Dr Sjoerd van Wijk from the Institute of Experimental Cancer Research in Paediatrics at Goethe University already two years ago found evidence indicating that the anti-diarrhoea drug loperamide could be used to induce cell death in glioblastoma cell lines.
They have now
deciphered its mechanism of action and, in doing so, are opening new avenues for
the development of novel treatment strategies.
When cells digest themselves
In certain types of tumour cells, administration of loperamide leads to a stress response in the endoplasmic reticulum (ER), the cell organelle responsible for key steps in protein synthesis in the body.
The stress in the ER triggers its degradation, followed by self-destruction of the cells. This mechanism, known as autophagy-dependent cell death occurs when cells undergo hyperactivated autophagy.
Normally, autophagy regulates normal metabolic processes and breaks down and recycles the valuable parts of damaged or superfluous cell components thus ensuring the cell’s survival, for example in the case of nutrient deficiency.
In certain tumour cells, however,
hyperactivation of autophagy destroys so much cell material that they are no
longer capable of surviving.
“Our experiments with cell lines show that autophagy could support the treatment of glioblastoma brain tumours,” says van Wijk.
Glioblastoma is a very aggressive and lethal type of cancer in children and adults that shows only a poor response to chemotherapy. New therapeutic approaches are therefore urgently required. The research group led by van Wijk has now identified an important factor that links the ER stress response with the degradation of the ER (reticulophagy):
The “Activating Transcription
Factor” ATF4 is produced in increased amounts both during ER stress and under
the influence of loperamide. It triggers the destruction of the ER membranes
and thus of the ER.
Anti-diarrhoea drug triggers cell death in glioblastoma
cells
“Conversely, if we block ATF4, far fewer cells in a tumour cell
culture die after adding loperamide,” says van Wijk, describing the control
results. In addition, the research group was able to detect ER debris in
loperamide-treated cells under the electron microscope. “ER degradation, that
is, reticulophagy, visibly contributes to the demise of glioblastoma cells,”
says van Wijk. The team also showed that loperamide triggers only autophagy but
not cell death in other cells, such as embryonic mouse fibroblasts. “Normally,
loperamide, when taken as a remedy against diarrhoea, binds to particular
binding sites in the intestine and is not taken up by the bowel and is
therefore harmless”.
Mechanism of action also applicable to other diseases
The loperamide-induced death of glioblastoma cells could help in the development of new therapeutic approaches for the treatment of this severe form of cancer.
“However, our findings also open up exciting new possibilities for the treatment of other diseases where ER degradation is disrupted, such as neurological disorders or dementia as well as other types of tumour,” says van Wijk.
However, further studies are necessary before loperamide can actually be used in the treatment of glioblastoma or other diseases. In future studies it has to be explored, for example, how loperamide can be transported into the brain and cross the blood-brain barrier. Nanoparticles might be a feasible option.
The research team in Frankfurt now wants to identify other substances
that trigger reticulophagy and examine how the effect of loperamide can be
increased and better understood.
The research group led by Sjoerd van Wijk is funded by the Frankfurt Foundation for Children with Cancer (Frankfurter Stiftung für krebskranke Kinder) and the Collaborative Research Centre 1177 “Molecular and Functional Characterisation of Selective Autophagy” funded by the German Research Foundation (Deutsche Forschungsgemeinschaft).
The work is the result
of collaboration with Dr Muriel Mari and Professor Fulvio Reggiori (University
of Groningen, The Netherlands) and Professor Donat Kögel (Experimental
Neurosurgery, Goethe University).
Publication: Svenja
Zielke, Simon Kardo, Laura Zein, Muriel Mari, Adriana Covarrubias-Pinto,
Maximilian N. Kinzler, Nina Meyer, Alexandra Stolz, Simone Fulda, Fulvio
Reggiori, Donat Kögel and Sjoerd van Wijk: ATF4 links ER stress
with reticulophagy in glioblastoma cells. Taylor & Francis
Online https://doi.org/10.1080/15548627.2020.1827780