Decellularized spinach serves as an edible platform for laboratory-grown meat
Boston College
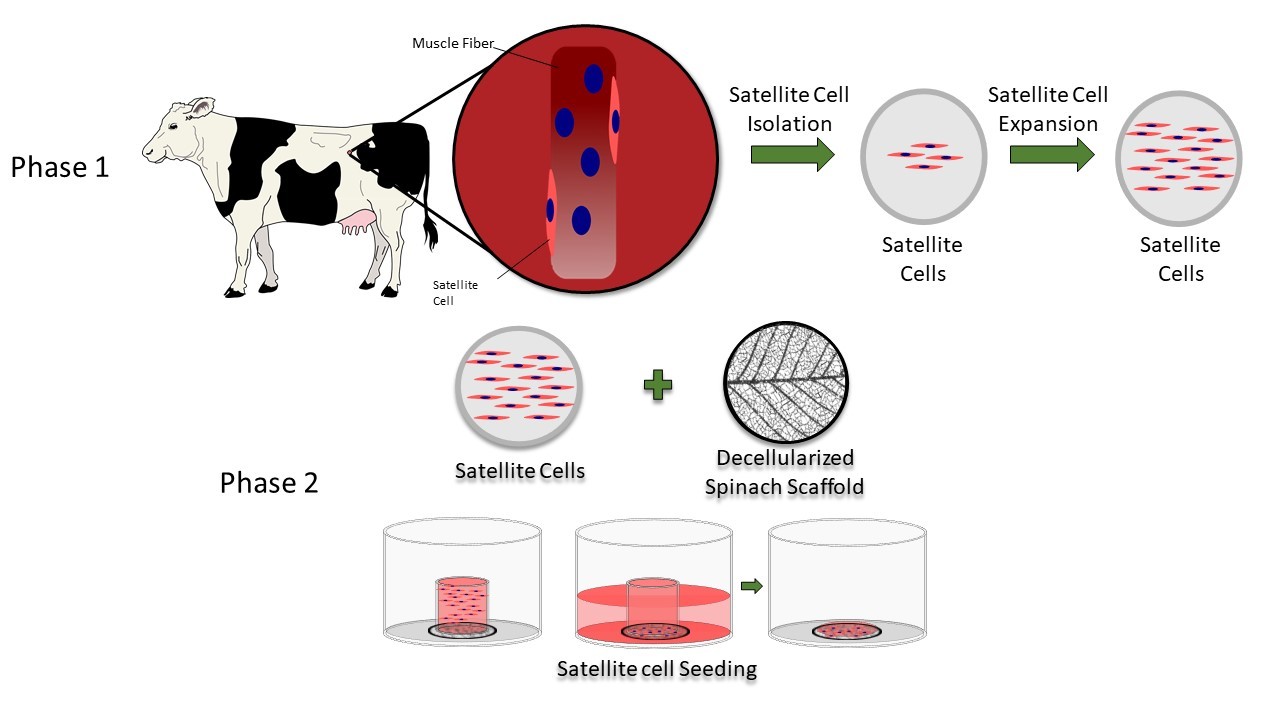 |
| This diagram shows the steps researchers took to isolate and seed primary bovine satellite cells on a decellularized spinach leaf scaffold. |
Spinach, a cost-efficient and environmentally friendly scaffold, provided an edible platform upon which a team of researchers led by a Boston College engineer has grown meat cells, an advance that may accelerate the development of cultured meat, according to a new report in the advance online edition of the journal Food BioScience.
Stripped
of all but its veiny skeleton, the circulatory network of a spinach leaf
successfully served as an edible substrate upon which the researchers grew
bovine animal protein, said Boston College Professor of Engineering Glenn
Gaudette, the lead author of the new study. The results may help increase the
production of cellular agriculture products to meet rising demand and reduce
environmental costs.
"Cellular agriculture has the potential to produce meat that replicates the structure of traditionally grown meat while minimizing the land and water requirements," said Gaudette, the inaugural chair of BC's new Engineering Department. "We demonstrate that decellularizing spinach leaves can be used as an edible scaffold to grow bovine muscle cells as they develop into meat."
Earlier
advances by Gaudette in this area garnered worldwide attention. In 2017, Gaudette
and a multi-university team showed that human heart tissue could be cultivated
on a spinach leaf scaffold, which was chosen because it offered a natural
circulatory system that is nearly impossible to replicate with available
scientific tools and techniques.
"In
our previous work, we demonstrated that spinach leaves could be used to create
heart muscle patches," said Gaudette. "Instead of using spinach to
regrow replacement human parts, this latest project demonstrates that we can
use spinach to grow meat."
Gaudette
said the team, which included Worcester Polytechnic Institute graduate students
Jordan Jones and Alex Rebello, removed the plant cells from the spinach leaf
and used the remaining vascular framework to grow isolated cow precursor meat
cells. The cells remained viable for up to 14 days and differentiated into
muscle mass.
"We need environmentally and ethically friendly ways to grow meat in order to feed the growing population," said Gaudette, whose research is supported by New Harvest.
"We set out to see if we can use an edible scaffold to accomplish
this. Muscle cells are anchorage dependent, meaning they need to grab on to
something in order to grow. In the lab, we can use plastic tissue culture
plates, but plastic is not edible."
The
researchers point out that the successful results will lead to further
characterization of the materials and scientific processes to better understand
how to meet consumer demand and gauge how large-scale production could be
accomplished in accordance with health and safety guidelines.
"We
need to scale this up by growing more cells on the leaves to create a thicker
steak," said Guadette. "In addition, we are looking at other
vegetables and other animal and fish cells."