URI researchers eradicate malignant tumors in mice
By Kevin Stacey
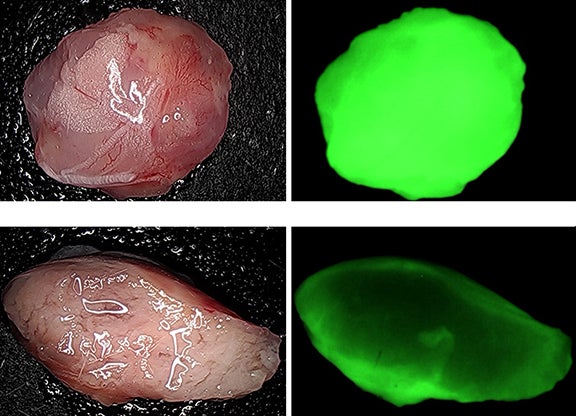
An untreated tumor (top left) is highly acidic, as indicated by its response to fluorescent dye (top right). Just days after treatment (bottom left), the tumor stroma is disrupted and pH decreases (bottom right).
Researchers from the University of Rhode Island and Yale University have demonstrated a promising new approach to delivering immunotherapy agents to fight cancer.
The approach involves tethering an immunotherapy agent
called a STING agonist to an acid-seeking molecule called pHLIP® (pH-low
insertion peptide). The pHLIP molecules target the high acidity of cancerous
tumors, delivering their immunotherapy cargo directly to cells in the tumor
microenvironment. Once delivered, the STING agonists engage the body’s innate
immune response to fight the tumor.
In a study published in Frontiers of Oncology, the team showed that just a single dose of the pHLIP-STING agonist combination eradicated colorectal tumors—even large, advanced tumors—in mice. The treated mice also developed lasting immunity, enabling their immune systems to recognize and fight cancer long after the initial tumors were gone.
While the researchers caution that
results in mice don’t always translate to humans, the findings do lay the
groundwork for a potential clinical trial testing the safety and effectiveness
in cancer patients.
“STING agonists are an important class of immuno-modulators, but research has clearly shown that they often don’t work on their own and need to be targeted in some way,” said Yana Reshetnyak, a physics professor at URI and a senior author of the new research. “What we show here is that using pHLIP to target tumors through their acidity, we can successfully go after a variety of different cell types within the tumor microenvironment and achieve synergistic and quite dramatic therapeutic effects.”
Targeted immunotherapy
Immunotherapy is an emerging approach to fighting
cancer. For cancer to survive and spread, tumors need to hide from the immune
system. In some cases, they do this by expressing proteins that act as immune
cloaking devices—tricking the immune system into thinking tumor cells are
normal, native cells. Immunotherapy aims to disable these cloaking devices.
One way of uncloaking tumors is through use of immune
checkpoint inhibitors, drugs that have proven effective in treating a variety
of cancers. But these drugs don’t work on all tumors. While they work well on
immunologically “hot” tumors with lots of inflammation, they are much less
effective in “cold,” non-inflamed tumors. STING (STimulator of InterferoN Gene)
agonists were developed as a means to turn cold tumors into hot ones—making
them more susceptible to an immune response. They do that by causing cells to
release interferon, a type of red-flag protein that alerts the immune system to
foreign invaders.
The approach has shown promise in the lab, but
administering STING agonist to patients has proven challenging, Reshetnyak
says. The compounds can affect healthy cells, leading to significant side
effects and only modest therapeutic effects.
If there were a way, however, to target STING agonists
specifically to tumor cells—not just cancer cells but also dormant immune cells
within a tumor—it may increase their effectiveness significantly. That’s where
pHLIP comes in.
PHLIP is a peptide (a chain of amino acids) derived
from bacteriorhodopsin, a membrane protein that enables some single-celled
organisms to convert light to energy. Research led by Donald Engelman at Yale
showed that pHLIP has a special affinity for acidic environments.
“When pHLIP encounters a cell membrane with a neutral
pH, it will sit on the surface briefly and then pull away,” said Engelman, who
is a co-author of this new study. “But if it’s in an acidic environment, then
the peptide folds into a helix, crosses the cell membrane and stays there.”
When Reshetnyak joined Engelman’s lab as a
postdoctoral researcher in 2003, she got the idea to try using this helix to
seek out cancer cells. It’s well known that malignant tumor cells tend to be
highly acidic. Along with Engelman and fellow URI physics professor Oleg
Andreev, Reshetnyak has been working for two decades to develop pHLIP as a
cancer-seeking delivery mechanism.
The team has shown that they can tether molecules to
the part of the pHLIP peptide that enters the cell membrane. Those cargo
molecules could be diagnostic agents that help doctors to see tumors more
clearly, toxins that kill cancer cells, or immuno-modulators like the STING
agonist. Because pHLIP only enters cells in highly acidic environments, they
can target tumor cells while leaving healthy cells alone.
There are currently two ongoing clinical trials
testing the safety of pHLIP compounds in cancer patients. And the team
continues to look for new ways of using the peptide. In this new study, the
researchers aimed to find out if pHLIP could successfully target
immunotherapeutic molecules that cause the immune system to attack tumors.
Eradicated tumors
To test whether targeting via pHLIP would increase the
effectiveness of STING agonist activity, the researchers gave 20 mice with
small colorectal tumors (100 cubic millimeters) a single injection of the
pHLIP-STING agonist. Within days, the tumors disappeared entirely in 18 mice.
The team also treated 10 mice with larger tumors (400 to 700 cubic millimeters)
with a single injection. Seven of those mice saw tumor eradication. For
comparison, 10 mice received injections of untargeted STING agonist. Tumors
remained in all mice, despite a modest slowing of growth for a short time.
The treatment also appears to have stimulated immune
memory in the treated mice. When cancer cells were injected in mice that had
been tumor-free for 60 days, new tumors failed to develop in those mice. That
suggests that once the immune system is primed to attack tumor cells, it
continues to do so without additional treatment.
The high rates of tumor eradication are encouraging,
the researchers say, but what’s also encouraging is the fact that the
pHLIP-STING agonist appears to be targeting multiple types of tumor cells.
Tumors contain more than just cancer cells. Many have a stroma, a kind of
coating of non-cancerous cells that forms both a physical and chemical barrier
that protects the tumor from the human immune system. In studying tumor
structure in the hours following pHLIP-STING agonist injection, the researchers
found a marked decrease in stromal cells.
“The stroma was essentially destroyed,” Reshetnyak
said. “The fact that we’re modulating the behavior of the wide variety of cells
in the tumor stroma as well as the cancer cells themselves means that we’re
inducing interferon signaling synergistically in multiple types of cells and
treating the entire tumor. That’s the advantage of using acidity as our target:
We’re able to go after the whole tumor rather than just certain cell types.”
There is more work ahead before a pHLIP-STING agonist treatment can be used in humans, the researchers say, but these preliminary results are promising. And because pHLIP-based treatments are already approved for clinical trials, the team hopes they will be able to move forward quickly.
The research was supported by the National Institutes of Health (R01 GM073857).
Engelman, Reshetnyak and Andreev are founders of pHLIP, Inc. and own shares in
the company. The University of Rhode Island and Yale have exclusively licensed
technology to pHLIP, Inc., which is commercializing pHLIP technology.