Big step forward in the fight against HIV/AIDS
Scripps Research Institute
While scientists have struggled in the past to create an effective vaccine against HIV, a novel vaccine design strategy being pursued by researchers at Scripps Research, IAVI, Fred Hutchinson Cancer Center (Fred Hutch) and the National Institutes of Health, National Institute of Allergy and Infectious Diseases (NIAID) Vaccine Research Center (VRC) shows new promise, according to data from a first-in-human clinical trial.
In a paper published in Science on December 2,
2022, the scientists reveal critical new insights into their novel vaccine
strategy, which involves a stepwise approach to producing antibodies capable of
targeting a wide range of HIV variants.
"The data we are publishing in Science demonstrates
for the first time that one can design a vaccine that elicits made-to-order
antibodies in humans. We specified in advance certain molecular properties of
the antibodies that we wanted to elicit, and the results of this trial show
that our vaccine antigen consistently induced precisely those types of
antibodies," says co-senior author William Schief, PhD, a professor and
immunologist at Scripps Research and executive director of vaccine design at
IAVI's Neutralizing Antibody Center, whose laboratory developed the vaccine
antigen. "We believe this vaccine design strategy will be essential to
make an HIV vaccine and may help the field create vaccines for other difficult
pathogens."
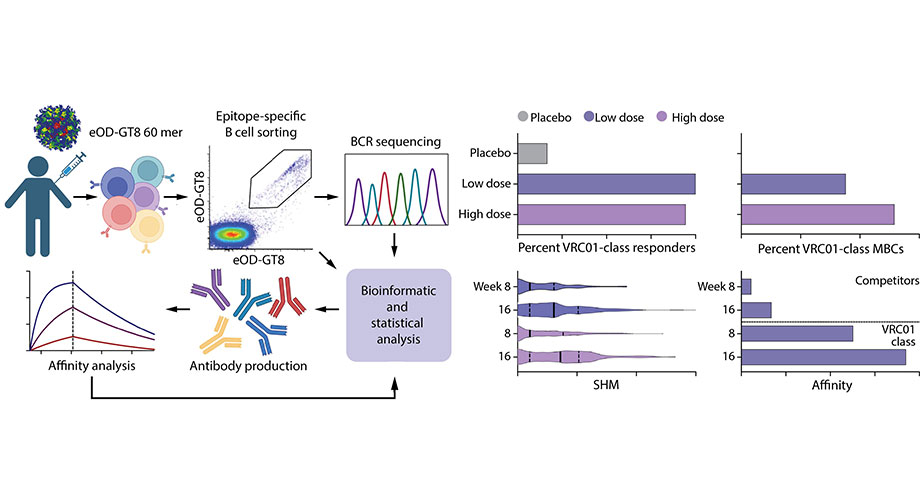
The Phase 1 trial, known as IAVI G001, tested the first stage in a multi-stage HIV vaccine regimen the researchers are developing. The trial results show that the vaccine had a favorable safety profile and induced the targeted response in 97% of people who were vaccinated. Importantly, the Science study also provides a detailed immunological analysis of the vaccine responses.
"HIV represents an area of dire unmet need across the
world, which is what makes the findings from our Phase 1 clinical trial so
encouraging," says Mark Feinberg, MD, PhD, president and CEO of IAVI.
"Through the close-knit collaboration of many different scientists,
disciplines and institutions, we are that much closer to designing an effective
vaccine that could help end the HIV pandemic."
Priming the Immune System
Broadly neutralizing antibodies (bnAbs) are a rare type of
antibody that can fight and protect against many different variants of a virus
-- including HIV. This is why scientists have tried to develop an HIV vaccine
that induces bnAbs, but thus far without success.
The researchers in the study are using a strategy known as
'germline targeting' to eventually produce bnAbs that can protect against HIV.
The first step of germline targeting involves stimulating the rare immune cells
-- known as bnAb-precursor B cells -- that can eventually evolve into the cells
that produce the bnAbs needed to block the virus. To accomplish this first
step, the researchers designed a customized molecule -- known as an immunogen
-- that would "prime" the immune system and elicit responses from
these rare bnAb-precursor cells.
The overarching goal of the IAVI G001 trial was to determine if
the vaccine had an acceptable safety profile and could induce responses from
these bnAb-precursor B cells.
"Through extensive safety and tolerability monitoring
during the trial, we showed the vaccine had a favorable safety profile, while
still inducing the necessary target cells," says study author Dagna
Laufer, MD, vice president and head of clinical development at IAVI. "This
represents a large step forward in developing an HIV vaccine that is both safe
and effective."
To determine if the targeted bnAb-precursor B cells were
induced, the researchers carried out a sophisticated analytical process.
"The workflow of multidimensional immunological analyses
has taken clinical trial evaluation to the next level," says co-senior
author Adrian B. McDermott, PhD, former chief of the Vaccine Immunology Program
at the NIAID VRC. "In evaluating these important immunological factors, we
helped show why the vaccine antigen was able to induce the targeted response in
97% of vaccine recipients."
IAVI G001 was sponsored by IAVI and took place at two sites:
George Washington University (GWU) in Washington, D.C., and Fred Hutch in
Seattle, enrolling 48 healthy adult volunteers. Participants received either a
placebo or two doses of the vaccine antigen, eOD-GT8 60mer, along with an
adjuvant developed by the pharmaceutical company GSK. Julie McElrath, MD, PhD,
co-senior author, senior vice president and director of Fred Hutch's Vaccine
and Infectious Disease Division, and David Diemert, MD, professor of medicine
at GWU School of Medicine and Health Sciences, were lead investigators at the
trial sites.
A Deeper Immunological Dive
The study also carefully examined the properties of the
antibodies and B cells induced by the vaccine antigen, in what Schief likens to
"looking under the car hood" to understand how the immune system
operated in response to the vaccine. One analysis showed that the vaccine
antigen first stimulated an average of 30 to 65 different bnAb precursors per
person vaccinated, and then caused those cells to multiply. This helped explain
why the vaccine induced the desired response in almost all participants.
Other analyses delved into the specific mutations the
bnAb-precursor B cells acquired over time and how tightly they bound to the
vaccine antigen. These investigations showed that that after each dose of the
vaccine, the bnAb-precursor B cells gained affinity and continued along
favorable maturation pathways.
One concern for this type of vaccine approach is the notion of
"competitors" -- in other words, the B cells induced by the vaccine
antigen that are not bnAb precursors. The researchers extensively studied the
"competitor" responses, and the results were very encouraging.
Although the majority of the B cells triggered by vaccination were, in fact,
"competitors," these undesired B cells could not match the binding
strength of the desired bnAb precursors and did not seem to impede maturation
of the bnAb-precursor responses.
"These findings were very encouraging, as they indicated
that immunogen design principles we used could be applied to many different
epitopes, whether for HIV or even other pathogens," adds Schief.
With these promising data in hand spanning both safety and
immune responses, the researchers will continue to iterate and design boosting
immunogens that could eventually induce the desired bnAbs and provide
protection against the virus. These findings also come shortly after two
additionalstudies in Immunity published in September 2022,
which helped validate the germline-targeting approach for vaccinating against
HIV.
"Working together with IAVI, Scripps Research, the VRC,
GWU, additional investigators at Fred Hutch and many others, this trial and
additional analyses will help inform design of the remaining stages of a
candidate HIV vaccine regimen -- while also enabling others in the field to
develop vaccine strategies for additional viruses," says McElrath of Fred
Hutch.
IAVI, Scripps Research, NIAID, the Bill & Melinda Gates
Foundation and the U.S. President's Emergency Plan for AIDS Relief (PEPFAR)
through the United States Agency for International Development (USAID) are
partnering with the biotechnology company Moderna to develop and test mRNA
delivery of these HIV vaccine antigens. Two Phase I clinical trials are
underway that build on IAVI G001, one (IAVI G002) at four sites in the U.S. and
another (IAVI G003) at the Center for Family Health Research in Kigali, Rwanda,
and The Aurum Institute in Tembisa, South Africa. Both are testing mRNA
delivery of the eOD-GT8 60mer that was evaluated as recombinant protein in IAVI
G001, and the U.S. trial includes a boost antigen designed by the Schief lab
and delivered with Moderna mRNA technology. A third trial (HVTN302), at ten sites
in the U.S., is testing mRNA delivery of three different stabilized HIV trimers
designed in the Schief laboratory that are candidates for late-stage boosters
in multi-stage vaccines aiming to induce bnAbs. Using mRNA technology could
significantly accelerate the pace of HIV vaccine development as it allows for
faster production of clinical trial material.
This work was supported by the Bill & Melinda Gates Foundation Collaboration for AIDS Vaccine Discovery; the IAVI Neutralizing Antibody Center; NIAID; Scripps Center for HIV/AIDS Vaccine Immunology and Immunogen Discovery and Scripps Consortium for HIV/AIDS Vaccine Development; and the Ragon Institute of MGH, MIT, and Harvard. Other collaborating organizations include Duke Human Vaccine Institute, Karolinska Institutet, and La Jolla Institute.