Speaking up for the annoying fruit fly
Andreas Prokop, University of Manchester
 |
| vasekk/Shutterstock |
Flies and mosquitoes both belong to Diptera, the group of insects that have only two wings (from the Greek di meaning two and pteron meaning wing). However, just as most people accept the bothersome as well as the positive traits of their friends, we shouldn’t judge flies for their negative behaviour alone.
We should open our eyes to their enormous economic and environmental importance, as entomologist Erica McAlister argues in her book The Secret Life of Flies. For example, many plants (including the cacao plant that gives us chocolate) rely on Diptera as pollinators. Or try to imagine a world without flies to decompose dead animals.
I will argue from a different angle, though, to win your respect for one specific dipteran: the fruit or vinegar fly (Drosophila melanogaster).
Drosophila may be smaller than a fingernail but it can be a big nuisance in summer when it hovers over maturing fruit or emerges in swarms from litter bins. The species Drosophila was first mentioned by German entomologist Johann Meigen in 1830 and has since earned a celebrity status among scientists.
It has become the best-understood animal organism on the planet and a powerhouse of modern medical research. Ten scientists working on Drosophila have been awarded a Nobel prize in physiology or medicine.
Science’s partnership with flies started during the early 1900s when biologist Thomas Hunt Morgan at Columbia University in New York decided to test evolutionary theories, such as how genetic mutations are linked to other characteristics, and the rediscovery in 1900 of Gregor Mendel’s theories of inheritance, published 1865. Mendel remains the acknowledged father of genetics today.
Helping science take off
Morgan was not the first to work with Drosophila. But his idea to harness the fly’s cheap husbandry (pieces of banana kept in milk bottles), and rapid reproduction (one generation in about ten days; about 100 eggs per female per day) would make it possible to study evolution in the laboratory. This is because it’s easier to see evolutionary changes in large populations of a species with high turnover.
His mass-breeding experiments with hundreds of thousands of flies led to the discovery of a single fly with white eyes, instead of the red eyes fruit flies normally have. Morgan and his team’s subsequent studies of its white-eyed progeny revealed that genes can mutate and are arranged into orderly and reproducible maps on chromosomes (a long DNA molecule). This new understanding founded the field of classical genetics as we know it. For example, it led to an understanding of how genetic disease is inherited.
In the 1940s, scientists, including George Beadle and Edward Tatum, established that some gene codes for proteins can facilitate chemical reactions and produce the molecules needed in cells.
Other researchers mapped the structure of the DNA helix and deciphered the genetic code. Through these developments, long-debated questions came into focus. For example, how genes regulate complex biological processes, such as the development of an entire organism from a single fertilised egg cell.
Scientists gradually established techniques using microscopes to study Drosophila embryos in their tiny 0.5mm transparent eggshells. The plethora of genetic strategies we’ve learned about in flies has turned into a powerful means to dissect mechanisms of fly development.
Just like human gene mutations can cause body malformations in people, fly embryos also show such defects. For example, lacking their heads or tails.
Scientists can study mutant defects, even if the eggs never hatch, which can then inform us about the normal function of the affected gene. These kinds of genetic studies of Drosophila, combined with emerging technologies, such as gene cloning, helped us understand how gene networks can determine the development of a body and how they can sometimes cause inherited disorders.
Gene networks are a set of genes, or parts of genes, that interact with each other to control a specific cell function. In 1995, three scientists won the Nobel prize for their contribution to this new understanding.
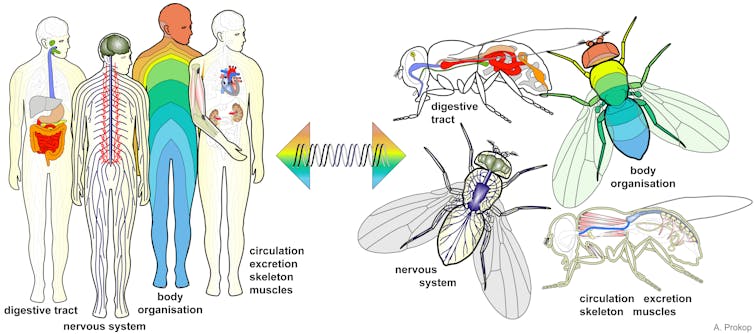 |
| Fruit flies and humans have surprisingly similar biology. Andreas Prokop, Author provided |
A startling likeness
Eventually, it emerged that the entire genomes of flies and humans showed astonishing similarities, and mechanisms or processes discovered in flies often turned out to apply to more complex organisms. Many human genes can even take over the function of their Drosophilia equivalent when inserted into the fly genome.
The common ancestor that founded the evolutionary lines of flies and humans, half a billion years ago, appears to have been equipped with biology so well-designed that many of its aspects are still maintained, such as mechanisms of growth or neuronal function.
Because we are so alike genetically, many aspects of human biology and disease have been explored first in Drosophila. Meanwhile, research on fruit flies is fast, cost-effective and extremely versatile. It’s ideal for scientific discoveries.
Once knowledge has been gained in a fly, that knowledge can accelerate research in more complex organisms. Today, over 10,000 researchers worldwide are estimated to work with Drosophila in many areas of science that relate to human biology and disease. It is used by neuroscientists for studying learning, memory, sleep, aggression, addiction and neural disorders. Not to mention cancer and ageing, processes of development, the gut microbiome, stem cells, muscles and the heart.
That said, flies are not mini-humans. They cannot be used to study personality loss seen in Alzheimer’s disease, for example. But they can be used to study why neurons die in such diseases and bridge important gaps in our understanding of this type of disease.
Fruit flies hovering in your kitchen might be aggravating, but hopefully you will see them in a different light now.![]() EDITOR'S NOTE: No.
EDITOR'S NOTE: No.
Andreas Prokop, Professor of Cellular and Developmental Neurobiology, University of Manchester
This article is republished from The Conversation under a Creative Commons license. Read the original article.