Here’s a Primer.
Many people have gotten used to rolling up their sleeves for flu and covid-19 vaccines.
New immunizations are also available to combat respiratory syncytial virus, or RSV, for those at high risk of illness. Although the one-time shots reached pharmacies last year, fewer than a quarter of those 60 or older nationally had been vaccinated as of May.
Even in Florida, not many older adults have gotten the shot yet. That’s telling for a place with a high concentration of seniors because, while the virus has traditionally been thought of as a childhood ailment that affects babies, older adults can suffer from it, too. And Florida, with its humid weather, is the nation’s ground zero for RSV.
Each year, infections typically start in Florida and the Southeast before spreading to other parts of the United States, according to the University of Florida’s Emerging Pathogens Institute.
The Sunshine State’s RSV season runs longer than anywhere else in the U.S., the institute said. There and in other places with tropical, humid climates, outbreaks can occur sporadically throughout most of the year, according to the institute.
That means RSV season is already underway in some parts of Florida and is coming soon to the rest of the country. Here’s what to know about it:
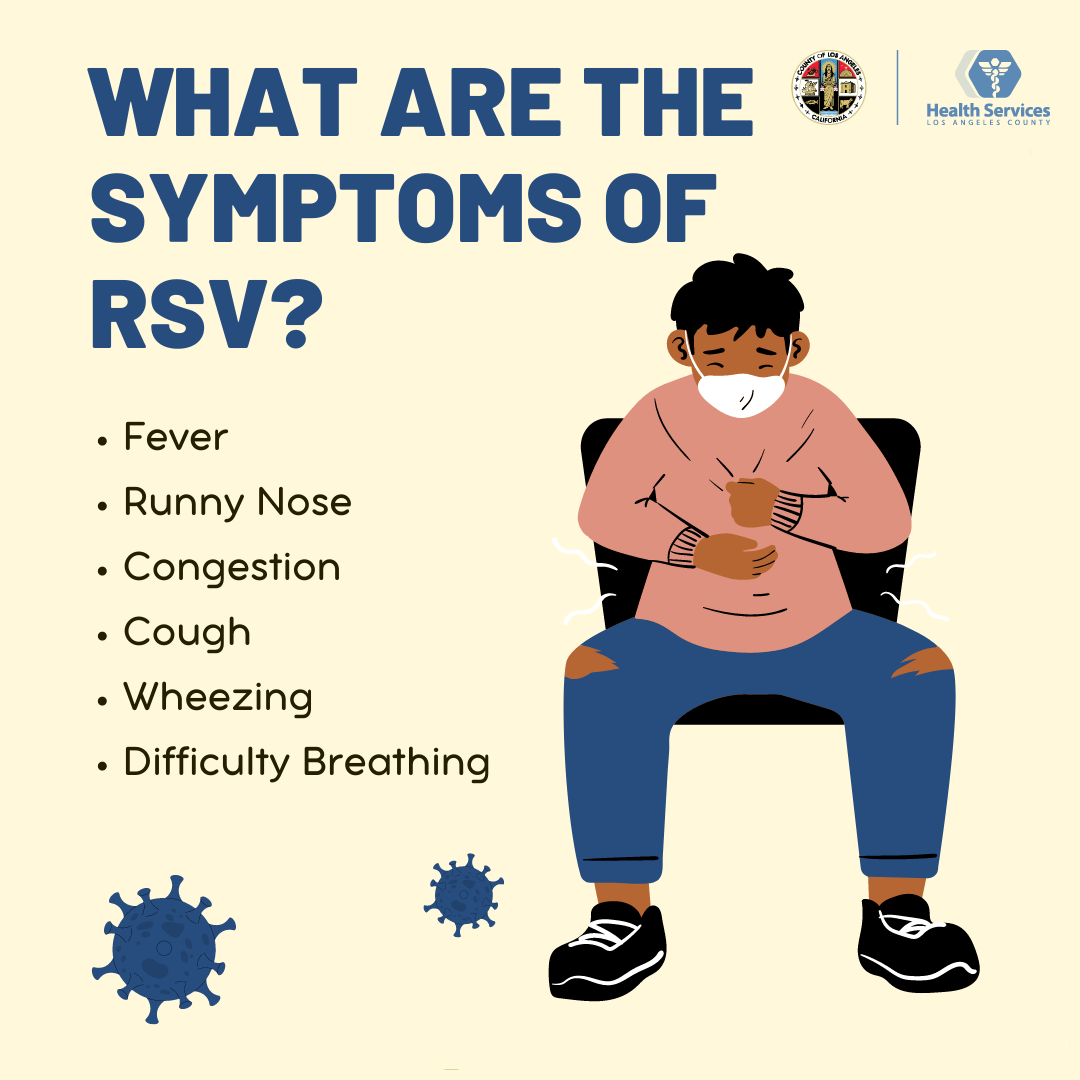
Q: What Is RSV?
The respiratory virus is common but gained more widespread recognition amid the covid pandemic.
In medical school, many doctors were taught that RSV was an important pediatric illness but not a major issue for older adults, said William Schaffner, an infectious diseases specialist at Vanderbilt University Medical Center in Tennessee.
The pathogen typically follows a seasonal pattern and usually causes mild cold-like symptoms, but it can lead to serious problems such as pneumonia in infants and seniors. It spreads through coughing, sneezing, direct contact, and contaminated surfaces.
An estimated 58,000 to 80,000 children under age 5 are hospitalized each year due to RSV in the United States, and as many as 300 die, according to the Centers for Disease Control and Prevention. Kids at highest risk include premature infants and those younger than 2 with chronic lung disease or congenital heart disease. RSV is the leading cause of infant hospitalization in the U.S.
Outbreaks of the virus can occur in settings like day care and schools.
Federal health officials also estimate that up to 160,000 older adults are hospitalized each year due to RSV and as many as 10,000 die.
For those infected, it can be a “really nasty and long-lasting type of illness,” said Nathaniel Hupert, co-director of the Cornell Institute for Disease and Disaster Preparedness at Weill Cornell Medicine in New York City.
In Florida, each of five regions has a slightly different period of heightened transmission. For example, central Florida’s runs from August to March.
Individual cases of RSV are not reportable to Florida health officials. Only outbreaks are. For now, RSV activity is steady in the Tampa Bay counties. BayCare Health System has seen “very few hospitalizations, especially for adults at this time,” said Laura Arline, its chief quality officer.
Q: What Vaccines Are Available?
In 2023, federal regulators signed off on RSV vaccines from pharmaceutical companies Pfizer and GSK. This year, they also green-lighted a shot from Moderna, which uses mRNA technology, as with its covid vaccine.
They also cleared an immunization called nirsevimab, an antibody drug, for use in babies last year.
Q: Who Is Eligible To Be Inoculated?
The CDC urges anyone age 75 or older to get vaccinated. The agency also recommends that those 60 to 74 at high risk of severe illness get the jab. That includes people with chronic heart or lung conditions and patients at nursing homes or other long-term care facilities.
Unlike with flu and covid, the RSV shot is not an annual immunization. Eligible seniors should receive the vaccine once. The best time to do so is in the late summer or early fall, the federal agency said.
To protect young children, health authorities recommend that pregnant mothers get Pfizer’s vaccine sometime during weeks 32 through 36 of pregnancy — with the dose administered from September to January — or that infants be immunized with an antibody drug.
Nirsevimab, developed by pharmaceutical companies AstraZeneca and Sanofi, is recommended for infants younger than 8 months during or entering their first RSV season. Another dose is urged for some kids ages 8 to 19 months who are at high risk and entering their second RSV season.
Nationally, an estimated 24% of those 60 or older reported getting an RSV vaccine during the initial rollout, and an additional 11% said they definitely planned to get it, according to CDC survey data from May.
Considering the lack of a universal health care system, and with many people having no primary care physician, “I think that this is a pretty impressive showing,” said Hupert of the Cornell Institute for Disease and Disaster Preparedness.
Q: How Much Do the Shots Cost?
The list price of GSK’s vaccine is $280 per dose. Pfizer’s is $295 per dose. Moderna’s retails for about the same, according to health care company GoodRx.
The amount people pay out-of-pocket is set by their insurance coverage’s prescription drug plan.
Under the Inflation Reduction Act of 2022, adults enrolled in Medicare Part D drug plans can receive federally recommended vaccines free, said Centers for Medicare & Medicaid Services spokesperson Lorraine Ryan in an email. If someone with Part D is having trouble obtaining RSV vaccine coverage, they should contact their plan or call 1-800-MEDICARE (800-633-4227) for assistance.
Q: Where Are the Vaccines Offered?
Doctor’s offices might stock them, but pharmacies or drugstores administered the majority of shots during the first season of availability.
Schaffner, of the Vanderbilt University Medical Center, said that’s because the vaccines are covered under Medicare Part D — not Part B. Many physicians “simply don’t deal with” Part D-covered immunizations, he said. Pharmacies do, though.
Q: Why Have So Few People Been Immunized?
Federal health officials last year said all adults 60 or older should have the option of getting a vaccine after discussing it with a health care provider. This is known as a “shared clinical decision-making” recommendation and has since been replaced with the latest guidance for those age 60 and beyond.
Shared clinical decision-making recommendations create financial and logistical barriers that can hinder vaccine uptake, according to the Champions for Vaccine Education, Equity + Progress, a coalition of patient, provider, and public health organizations.
Many physicians didn’t know about the shots during the first season of availability and weren’t convinced of their importance, so further education was needed, Schaffner added. A lot were cautious, too, he said, because of reports of a rare nervous system disorder, Guillain-Barré syndrome, occurring in a small share of people post-vaccination.
Advisers to the CDC concluded that the estimated benefits of getting an RSV vaccine outweigh the risks.
“Let’s see if we can do better this second year,” Schaffner said.
This article was produced through a partnership between KFF Health News and the Tampa Bay Times.
KFF Health News is a national newsroom that produces in-depth journalism about health issues and is one of the core operating programs at KFF—an independent source of health policy research, polling, and journalism. Learn more about KFF.Subscribe to KFF Health News' free Morning Briefing.